U of T researchers develop tiny 'robotic hand' for minimally invasive brain surgery
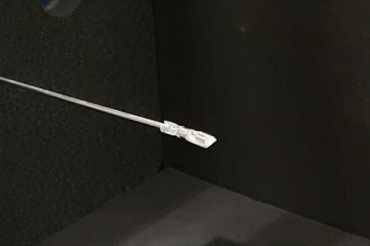
A surgical tool developed by U of T Engineering researchers uses magnetic fields to enable surgeons to access hard-to-reach areas of the brain with a minimal level of invasiveness (video via Microrobotics Lab)
Published: May 25, 2023
A tiny robotic hand designed to enhance neurosurgery is one step closer to clinical practice.
The microrobotic tool is created by a team of University of Toronto researchers led by Eric Diller, an associate professor in the department of mechanical and industrial engineering in the Faculty of Applied Science & Engineering.
Operated by an electromagnetic system, the device enables surgeons to access hard-to-reach areas of the brain with a minimal level of invasiveness, promising faster treatment and recovery for patients.
“We are designing the mechanism that drives this robotic hand, which is basically going to act as a surgeon’s hand,” Diller says. “We are also using magnetic fields to make this tiny hand move, which is our unique approach to doing this.”
The team will be presenting their latest findings at the 2023 IEEE International Conference on Robotics and Automation (ICRA) later this spring. Their new conference paper examines the feasibility of the newly developed tools to ensure they are ready for preclinical trials.
“No one else has developed these wirelessly driven magnetic tools before,” says Diller. “So, we needed to categorize the different types of basic operation elements that a surgeon would do, such as pulling on tissue, retracting and applying force to cut into the tissue.
“We determined that for brain surgery – including procedures targeting epilepsy or removing tumours – we can get enough force to perform the necessary neurosurgery tasks.”
The designs presented in the new study are an extension of two previous papers published in 2021 in collaboration with James Drake, surgeon-in-chief at The Hospital for Sick Children (SickKids) and a professor of surgery in U of T’s Temerty Faculty of Medicine.
Since then, the team has developed a clinical-scale electromagnetic coil system, which was designed and built by U of T Engineering alumnus Adam Schonewille, a former student in Diller’s lab.
The system has a working volume that is approximately the size of an adult human head, with all the electromagnets located underneath a flat surface – a design requirement for Drake’s team at SickKids, since surgeons require unimpeded access to the patient.
“Existing surgical robots already take up a lot of space in the operating room, so we wanted our system to be as unobtrusive as possible while still giving the magnetic field the strength needed to accomplish the work,” says Cameron Forbrigger, who earned his PhD from U of T Engineering last year and is lead author of the new paper.
“This electromagnetic system is a major step forward for the feasibility of our surgical approach, and we’ve seen a lot of interest in it from international researchers in our field.”
A significant contribution of Forbrigger’s PhD dissertation involved modelling how the magnetic design of a tool shapes its response to the magnetic field. Using that model, he was able to rank tool designs based on their predicted performance.
“This accelerates our design process because we don’t need to build a tool and test it to know how it will behave,” he says. “This model also enabled us to develop a control strategy that automatically calculates the optimal magnetic field needed to move the tool through a desired motion.”
The team is also working to overcome a significant challenge that many surgical robots face: acquiring real-time information about the tool’s location and orientation.
Surgeons using the tool will need to insert it down a channel into the brain and know where it is. To simulate this, the research team makes use of “phantom” brains made of rubber, inserting the long, thin tool into the model that is the same size and shape as a real brain.
While the camera on the tip of the tool provides some location information, Diller says the feedback isn’t very accurate due to its poor viewpoint. To overcome this visual challenge, PhD candidate Erik Fredin, the second author on the conference paper, is developing a computer vision algorithm using machine learning, which is crucial for the utility of the tool. The computer vision results show that it can detect the angles of the tool as the operator controls it.
The next step toward clinical use and commercialization will be moving the electromagnetic system and tools to SickKids hospital for live animal trials.
“Surgeons can be skeptical about the effectiveness of a new surgical tool until they see it tested in a realistic scenario – and rightfully so,” says Forbrigger, who is now a post-doctoral researcher at ETH Zürich.
“We’ve put a lot of effort into demonstrating the performance of the tools quantitatively, but we’ve now reached the point where animal models are the next critical step toward further development.”



